At the primary school level, typically people are taught that there are three states of matter: solid, liquid, and gas. (Plasma may be introduced as a fourth state sometimes.) These three states are readily distinguished because they have vastly different mechanical properties. We now know that there are many more states of matter than just those few, because we have developed ways to look at materials that can see differences that are much more subtle than bulk mechanical response. As I discussed a little bit here, something is a "solid" if it resists being compressed and sheared; the constituent atoms/molecules are right up against each other, and through their interactions (chemical bonds, "hard-core repulsion"), the material develops internal stresses when it's deformed that oppose the deformation.
Broadly speaking, there are two kinds of solids, crystals and glasses. In crystals, which physicists love to study because the math is very pretty, the constituent atoms or molecules are spontaneously arranged in a regular, repeating pattern in space. This spatial periodicity tends to minimize the interaction energy between the building blocks, so a crystalline structure is typically the lowest energy configuration of the collective bunch of building blocks. The spatial periodicity is readily detectable because that repeating motif leads to constructive interference for scattering of, e.g., x-rays in particular directions - diffraction spots. (Most crystalline solids are really polycrystalline, an aggregation of a bunch of distinctly oriented crystal grains with boundaries.)
The problem is, just because a crystalline arrangement is the most energetically favored situation, that doesn't mean that the building blocks can easily get into that arrangement if one starts from a liquid and cools down. In a glass, there are many, many configurations of building blocks that are local minima in the potential energy of the system, and the energy required to change from one such configuration to another is large compared to what is available thermally. A paper on this is here. In ordinary silica glass, the local chemistry between silicon and oxygen is the same as in crystalline quartz, but the silicon and oxygen atoms have gotten hung up somehow, kinetically unable to get to the crystalline configuration. The glass is mechanically rigid (on typical timescales of interest - glass does not meaningfully flow). Try to do x-ray diffraction from a glass, and instead of seeing the discrete spots that you would with a crystal, instead you will get a mushy ring indicating an average interparticle distance, like in a liquid (when the building blocks are also right up against each other).
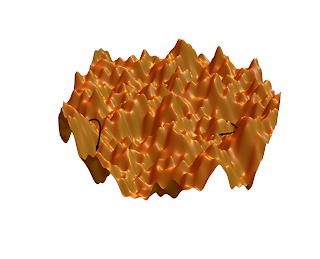 |
| Figure (credit: Chiara Cammarota, from here): A schematic rugged energylandscape with a multitude of energy minima, maxima, and saddles. Arrows denote some of the possible relaxation pathways. |
A hallmark of glasses is that they have a very broad distribution of relaxation times for structural motions, stretching out to extremely long timescales. This is a signature of the "energy landscape" for the different configurations, where there are many local minima with a huge distribution of "barrier heights". This is illustrated in the figure at right (sourced from the Simons Collaboration on Cracking the Glass Problem). Glasses have been a fascinating physics problem for decades. They highlight challenges in how to think about thermodynamic equilibrium, while having universality in many of their properties. Window glass, molecular glasses, many polymers that we encounter - all of these disparate systems are glasses.